Abstract
Background: Almost two thirds of transplant-ineligible, treatment naïve multiple myeloma (NDMM, TNE) patients (pts) do not proceed to second line anti-MM therapy. Given depth of response to initial therapy correlates to overall survival (OS), a deep remission should also be the target for this cohort of generally elderly and frail patients. However, this should not come at the expense of either treatment-related or fiscal toxicity. IRIL is a phase II, multicentre, response-adapted study examining treatment intensification with isatuximab (Isa; supported by Sanofi/Genzyme), an anti-CD38 monoclonal antibody, for pts not achieving pre-defined target responses to lenalidomide and dexamethasone (Rd).
Method: TNE NDMM pts meeting IMWG criteria for treatment are eligible for enrolment. Pts commence treatment with lenalidomide [25mg D1-D21 of a 28-day cycle (C)] and dexamethasone [40mg (20mg for those aged ³75 years) PO weekly]. Failure to achieve pre-defined target response [<PR after 4 cycles, <VGPR after 6, or <CR after 9 cycles of Rd] or progressive disease (PD) within the first 9 cycles of Rd leads to addition of Isa (10mg/kg IV weekly for cycle 1, then fortnightly) until PD or adverse events (AEs) that warrant treatment cessation. The primary endpoint is to evaluate the rate of achievement of ³PR following completion of 6 cycles Isa-Rd in those who failed to achieve <PR after C4 Rd. The secondary endpoints are to evaluate overall improvement in depth of response to Isa-Rd, progression free survival (PFS), OS, and safety.
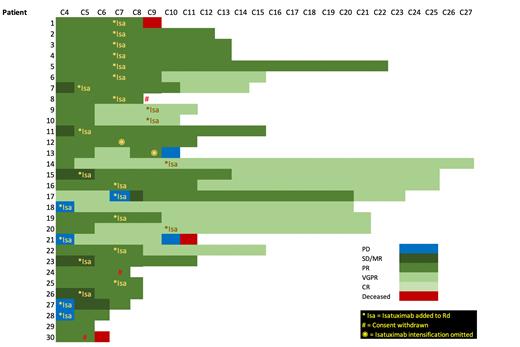
Results: From June 2019 to June 2021, 42 pts [52.3% male, median age 77.7 yrs (range 68.5-86.0), R-ISS Stage I (n=8), Stage II (n=23) and Stage III (n=7)] were accrued. 30 pts have completed at least C4 Rd and were deemed evaluable (12 non-evaluable; 5 only recently recruited, 5 withdrew consent (WD) due to logistic reasons, 2 PD pre-C4 and were taken of study at investigator discretion). Of the 30 evaluable pts (see Figure 1), 25 remain on study with 2 further WD (logistic reasons) and 3 deaths. In total, 25pts have had treatment intensification with Isa [9pts (5<PR and 4 PD) prior to C4 Rd, 11/13 eligible < VGPR after C6 Rd (1 omission in error, 1 pt WD consent), 4/5 eligible pts < CR after C9 Rd (1 omission in error) and 1 PD at C6]. 18/25 patients have had at least 6 months of Isa intensification with increased depth of response in 12 (66.7%) pts. Of the 9 pts rescued with Isa after not reaching target response post C4 Rd, 7 have completed 6 Isa-Rd cycles with 100% deepening of response (5 PR, 4 VGPR). The overall response rate in the cohort of evaluable patients is 100% (16 PR, 14 VGPR). The median (±SD) follow-up time of the evaluable cohort was 9.96 ± 6.24 months with med OS not reached. Thirty-one (73.8%) pts experienced any grade AE (median = 6; range 1-21). Grade 3 or 4 AEs were reported in 16 pts (34 events in total; median per pt = 2; range 1-5). Most common ³ Grade 3 events include infection (10), neutropenia (7) and insomnia and mood disorder (3 each). Neutropenia was the single grade 4 AE in a patient on Rd. Fewer of the reported grade 3 or 4 AEs occurred while on Isa-Rd (16) than while on Rd alone (n=18) with causality was less frequently attributed to Isa (n=4) than R (n=17) or d (n=14).
Conclusion: A response-adapted approach for TNE NDMM pts with isatuximab intensification upon inadequate response to standard-of-care lenalidomide-dexamethasone is both safe and effective. Isa-Rd leads to universal deepening of response in patients failing to achieve a PR or better after 4 months of Rd, while the overall response rate in evaluable patients, irrespective of initial response to Rd, is 100%. Isa-Rd is well tolerated in this elderly patient cohort. The safety profile for the combination Isa-Rd is similar to previous reports. Patient accrual is ongoing.
Figure 1: Swimmer's plot highlighting patient response after C4 Rd, timepoints of isatuximab intensification (*Isa) and subsequent depth and duration of response.
Janowski: Astrazeneca: Membership on an entity's Board of Directors or advisory committees; Regeneron: Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Spencer: Celgene: Honoraria, Research Funding, Speakers Bureau; Janssen: Honoraria, Research Funding, Speakers Bureau; Amgen: Honoraria, Research Funding; Bristol Myers Squibb: Research Funding; Takeda: Honoraria, Research Funding, Speakers Bureau; STA: Honoraria. Quach: Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Antengene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen/Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL: Consultancy, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal